
- Health
- Lead News
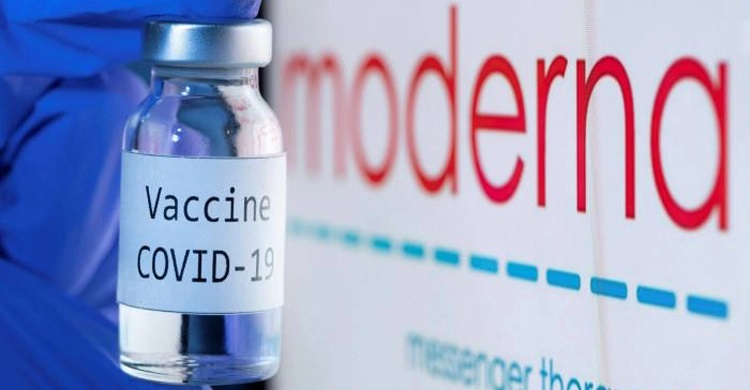
Moderna expects the vaccine trial data for kids aged 2 to 5 in March
- Health
- Lead News
- 14 January, 2022 03:46:26
CNI Desk: Moderna said on Wednesday (Jan 13) it expects to report data from its COVID-19 vaccine trial in children aged between 2 to 5 years in March.
“If the data is supportive and subject to regulatory consultation, Moderna may proceed with regulatory filings for children 2-5 years of age thereafter,” the company said.
Moderna’s vaccine, based on the messenger RNA platform, already has authorisations in Europe, UK, Australia, and Canada for adolescents aged 12 to 17 years, and has submitted applications for children in 6 to 11 years.
In the United States, the vaccine is authorised by Food and Drug Administration as primary two-dose regimen and booster dose for adults 18 years and older. The company, however, is yet to get an authorisation from the regulator for use of its vaccine in children.





















Comment ( 0)